Another principle, the PRINCIPLE OF THE
CONSERVATION OF MATTER, states that
matter can be neither created nor destroyed, but
only transformed. As you probably know, the
development of the atom bomb demonstrated that
matter can be converted into energy; other
developments have demonstrated that energy can
be converted into matter. Therefore, the principle
of the conservation of energy and the principle
of the conservation of matter are no longer
considered as two parts of a single law or principle
but are combined into one principle. That
principle states that matter and energy are
interchangeable, and the total amount of energy
and matter in the universe is constant.
The interchangeability of matter and energy
is mentioned here only to point out that the
statement energy in must equal energy out is not
strictly true for certain situations. However, any
noticeable conversion of matter into energy or
energy into matter can occur only under very
special conditions, which we need not consider
now. All the energy transformations that we will
deal with can be understood quite simply if we
consider only the principle of the conservation of
energy—that is,
ENERGY IN EQUALS
ENERGY OUT.
Transformation of Heat to
Work (Laws of Gases)
The energy transformation from heat to work
is the major interest in the shipboard engineer-
ing plant. To see how this transformation occurs,
we need to consider the pressure, temperature,
and volume relationships that hold true for gases.
Robert Boyle, an English scientist, was among
the first to study the compressibility of gases. In
the middle of the 17th century, he called it the
“springiness” of air. He discovered that when the
temperature of an enclosed sample of gas was kept
constant and the pressure doubled, the volume
was reduced to half the former value. As the
applied pressure was decreased, the resulting
volume increased. From these observations he
concluded that for a constant temperature, the
product of the volume and pressure of an enclosed
gas remains constant. This conclusion became
Boyle’s law.
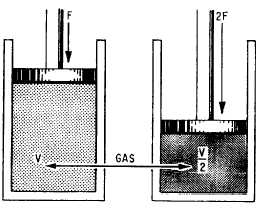
You can demonstrate Boyle’s law by confining
a quantity of gas in a cylinder that has a tightly
fitted piston. Apply force to the piston to
compress the gas in the cylinder to some specific
volume. If you double the force applied to the
Figure 2-5.—Compressibility of gas.
piston, the gas will compress to one half its
original volume (fig. 2-5).
Changes in the pressure of a gas also affect
the density. As the pressure increases, its volume
decreases; however, no change occurs in the
weight of the gas. Therefore, the weight per unit
volume (density) increases. So, the density of a
gas varies directly as the pressure if the
temperature is constant.
In 1787, Jacques Charles, a Frenchman,
proved that all gases expand the same amount
when heated 1 degree if the pressure is kept
constant. The relationships that these two men
discovered are summarized as follows:
l Boyle’s law—when the temperature is held
constant, an increase in the pressure on a gas
causes a proportional decrease in volume. A
decrease in the pressure causes a proportional
increase in volume, as shown in figure 2-6. At sea
level, the balloon has a given volume with respect
to temperature and atmospheric pressure. As the
balloon descends 1 mile below sea level, the
volume of the balloon decreases due to increased
atmospheric pressure. Conversely, as the balloon
ascends to 1 mile above sea level, the balloon
expands as the atmospheric pressure decreases.
l Charles’s law—when the pressure is held
constant, an increase in the temperature of a gas
causes a proportional increase in volume. A
decrease in the temperature causes a proportional
decrease in volume, as shown in figure 2-7.
Balloons A and B have an outside pressure of 10
pounds per square inch (psi). Both have the same
volume of air. Balloon A is at 40°F and balloon
B is at 100°F. This shows that increased
temperature causes the balloon size to increase.
2-10