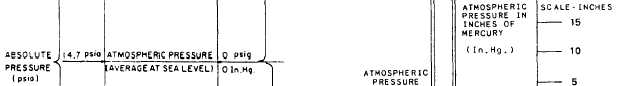
Figure 2-11.—Relationships among gauge pressure, atmos-
pheric pressure, vacuum, and absolute pressure.
the word pressure, the relationships among gauge,
atmospheric, vacuum, and absolute pressures are
shown in figure 2-11.
GAUGE PRESSURE is the pressure actually
shown on the dial of a gauge that registers
pressure relative to atmospheric pressure. An
ordinary pressure gauge reading of zero does
not mean there is no pressure in the absolute
sense; rather, it means there is no pressure in
excess of atmospheric pressure.
ATMOSPHERIC PRESSURE is the pressure
exerted by the weight of the atmosphere. At sea
level, the average pressure of the atmosphere is
sufficient to hold a column of mercury at the
height of 76 centimeters or 29.92 inches. Since a
column of mercury 1 inch high exerts a pressure
of 0.49 pound per square inch (psi) at its base,
a column of mercury 29.92 inches high exerts a
pressure that is equal to 29.92 x 0.49 or about 14.7
psi. Since we are dealing now in absolute pressure,
we say that the average atmospheric pressure at
sea level is 14.7 pounds per square inch absolute
(psia). It is zero on the ordinary pressure gauge.
Notice, however, that the figure of 14.7 psia
represents the average atmospheric pressure at sea
level; it does not always represent the actual
pressure being exerted by the atmosphere at the
moment a gauge is being read. Since fluctuations
from this standard are shown on a barometer
(an instrument used to measure atmospheric
pressure), the term barometric pressure is used to
Figure 2-12.—Typical barometer.
describe the atmospheric pressure that exists at
any given moment. Figure 2-12 shows the
operating principle of a typical barometer.
BAROMETRIC PRESSURE is the term used
to describe the actual atmospheric pressure that
exists at any given moment. Barometric pressure
may be measured by a simple mercury column or
by a specially designed instrument called an
aneroid barometer.
A space in which the pressure is less than
atmospheric pressure is said to be under partial
vacuum. The vacuum is expressed in terms of the
difference between the absolute pressure in the
space and the pressure of the atmosphere. Most
commonly, vacuum is expressed in inches of
mercury, with the vacuum gauge scale marked
from 0 to 30 in.Hg. When a vacuum gauge reads
zero, the pressure in the space is the same as
atmospheric pressure—or, in other words, there
is no vacuum. A vacuum gauge reading of 29.92
in. Hg would indicate a perfect (or nearly perfect)
vacuum. In actual practice a perfect vacuum is
impossible to obtain even under laboratory
conditions. A reading between 0 and 29.92 in.Hg
is a partial vacuum.
ABSOLUTE PRESSURE is atmospheric
pressure plus gauge pressure, or absolute pressure
minus vacuum. For example, a gauge pressure of
300 pounds per square inch gauge (psig) equals
an absolute pressure of 314.7 psia (300 + 14.7).
Or, for example, consider a space in which the
measured vacuum is 10 in. Hg; the absolute
2-16