next paragraph). In a compound the molecule is
the smallest part that has all the characteristics
of the compound. Consider water, for example.
Depending on the temperature, it may exist as a
liquid (water), a solid (ice), or a gas (steam).
Regardless of the temperature, it will still have
the same composition. If we start with a quantity
of water, divide this and pour out one half, and
continue this process enough times, we will end
up with a quantity of water that cannot be
further divided without ceasing to be water. This
quantity is called a molecule of water. If this
molecule of water is divided, instead of two parts
of water, we will have one part of oxygen and two
parts of hydrogen (H2O).
ATOMS
Molecules are made up of smaller particles
called ATOMS. An atom is the smallest particle
of an element that retains the characteristics of
that element. The atom of one element, however,
differs from the atoms of all other elements, Since
over 100 elements are known, there must be over
100 different atoms, or a different atom for each
element. Just as thousands of words are made by
a combination of the proper letters of the
alphabet, so thousands of different materials
are made by the chemical combination of the
proper atoms. Any particle that is a chemical
combination of two or more atoms is called a
molecule. The oxygen molecule has two atoms of
oxygen, and the hydrogen molecule has two
molecules of hydrogen. Sugar, on the other hand,
is a compound composed of atoms of carbon,
hydrogen, and oxygen. These atoms are combined
into sugar molecules. Since the sugar molecules
can be broken down by chemical means into
smaller and simpler units, we cannot have sugar
atoms.
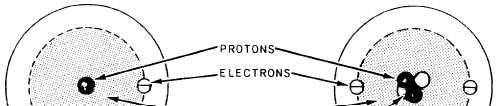
In figure 2-1 you will see that the atoms of
each element are made up of electrons, protons,
and,
in most cases, neutrons, which are
collectively called subatomic particles. Further-
more, the electrons, protons, and neutrons of one
element are identical to those of any other
element. The reason there are different elements
is that the number and arrangement of electrons
and protons within the atom are different for the
different elements.
The electron is considered to be a small
negative charge of electricity. The proton has a
positive charge of electricity equal and opposite
to the charge of the electron. Scientists have
measured the mass and size of the electron and
proton. They know how much charge each has.
The electron and proton each have the same
quantity of charge, although the mass of the
proton is about 1837 times that of the electron.
In some atoms, a neutral particle exists called a
neutron. The neutron is a mass about equal to
that of a proton, but it has no electrical charge.
According to a popular theory, the electrons,
protons, and neutrons of the atoms are thought
to be arranged in a manner similar to a miniature
solar system. The protons and neutrons form a
heavy nucleus with a positive charge, around
which the very light electrons revolve.
Figure 2-1.—Structure of simple atoms.
2-2