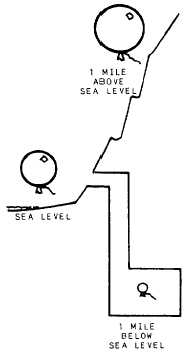
Figure 2-6.—Pressure differential in respect to sea level.
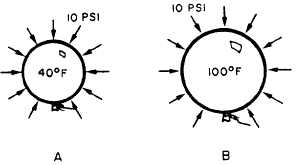
Figure 2-8.—Interaction of gases in respect to temperature
and pressure.
Suppose we have a boiler in which steam has
been formed. With the steam stop valves still
closed, the volume of the steam remains constant
while the pressure and the temperature are both
increasing. When operating pressure is reached
and the steam stop valves are opened, the high
pressure of the steam causes the steam to flow to
the turbines. The pressure of the steam thus
provides the potential for doing work. The actual
conversion of thermal energy to work is done in
the turbine section.
Steam
Steam is water to which enough heat has been
Figure 2-7.—Pressure differential in respect to temperature.
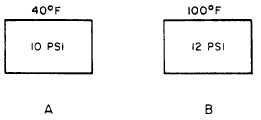
l Charles’s law is also stated—when the
volume is held constant, an increase in the
temperature of a gas causes a proportional
increase in pressure. A decrease in the temperature
causes a proportional decrease in pressure, as
shown in figure 2-8. Tanks A and B are of the
same size and have an equal volume of gas. Tank
A has a pressure of 10 psi when heated to 40°F.
Tank B has a pressure of 12 psi when heated to
100°F. Unlike the balloons, the steel tanks do not
expand to accommodate the changes in tempera-
ture and pressure. This shows that changes in
temperature are inversely proportional to changes
in gas pressure when the volume is held constant.
added to convert it from the liquid to the gaseous
state. When heat is added to water in an open
container, steam forms. However, it quickly mixes
with air and cools back to water that is dispersed
in the air, making the air more humid. If you add
the heat to water in a closed container, the steam
builds up pressure. If you add exactly enough heat
to convert all the water to steam at the
temperature of boiling water, you get saturated
steam. SATURATED STEAM is steam saturated
with all the heat it can hold at the boiling
temperature of water.
The boiling temperature of water becomes
higher as the pressure over the water becomes
higher. Steam hotter than the boiling temperature
of water is called SUPERHEATED STEAM.
When steam has 250 °F of superheat, the actual
temperature is the boiling temperature plus 250 °F.
At 600 psi the boiling temperature of water is
489 °F. So if steam at 600 psi has 250°F of
superheat, its actual temperature is 739°F. WET
STEAM is steam at the boiling temperature that
still contains some water particles. DESUPER-
HEATED STEAM is steam that has been cooled
by being passed through a pipe extending through
2-11