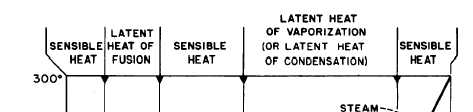
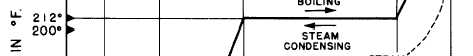
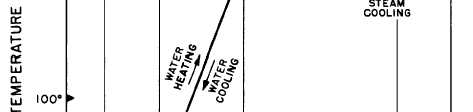
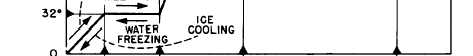
Figure 2-9.—Relationship between sensible heat and latent heat.
sensible heat and latent heat, we are talking about
The energy price is repaid, so to speak, when the
two different kinds of effects that can be produced
by heat, but not about two different types of heat.
As previously discussed, the three basic
physical states of all matter are solid, liquid, and
gas (or vapor). The physical state of a substance
is closely related to the distance between
molecules. As a general rule, the molecules are
closest together in solids, farther apart in liquids,
and farthest apart in gases. When heat flow to
a substance is not reflected in a temperature
increase in that substance, the energy is being
used to increase the distance between the
molecules of the substance and to change it from
a solid to a liquid or from a liquid to a gas. You
might say that latent heat is the energy price that
must be paid for a change of state from solid to
liquid or from liquid to gas. The energy is not lost.
It is stored in the substance as internal energy.
substance changes back from gas to liquid or from
liquid to solid, since heat flows from the substance
during these changes of state.
Figure 2-9 shows the relationship between
sensible heat and latent heat for water at
atmospheric pressure. The same kind of chart
could be drawn for other substances; however,
different amounts of thermal energy would be
involved in the changes of state for each
substance.
If we start with 1 pound of ice at 0°F, we must
add 16 Btu to raise the temperature of the ice to
32°F. We call this adding sensible heat. To change
the pound of ice at 32°F to a pound of water at
32°F, we must add 144 Btu (the LATENT HEAT
OF FUSION). No change in temperature will
occur while the ice is melting. After all the ice has
2-13